L-01-Restriction digestion with Block Cooler as incubation system
MATERIAL
- 10 U/µl EcoRI (Fermentas, Glen Blunie, MD, USA) and
- 10X EcoRI buffer (Fermentas)
- Nuclease Free Water (New England Biolabs, Ipswich, MA, U.S.A.)
- 0.17 µg/µl lambda DNA completely digested by HindIII (Wealtec, Taipei, Taiwan)
- 5X DNA sample loading dye (Wealtec)
- Agarose (item no: LA10101; Wealtec)
- 10X TBE solution (Bio-Rad, Hercules, CA, U.S.A.)
- 500 bp DNA ladder (GeNeI, Bangalore, India)
- 1 Kb DNA ladder (New England biolabs)
- 10 mg/ml EtBr (Bio-Rad)
- GES-equipment (gel tray, dams claws, comb) (Wealtec)
- Dolphin-Doc Plus Image system (Wealtec)
PROCEDURE
Block cooler temperature setting:
- The temperature of the block cooler was set 30 minutes prior to usage in order to stabilise the temperature.
- Temperature setting (fig. 2): The block cooler main switch on the back of the module was turned on, and then the “up”-arrow button above “MODE” was pressed three times to reach pre-setting “37°C”. For fine-tuning or manual temperature setting, use the up/down arrows above “TEMPERATURE”
- Thereafter, the “RUN/STOP”-button (fig. 2) was pressed in order to start the warm-up. The display shows the set temperature as well as the current temperature of the cooler (fig. 3).
- The red lamp indicates that the system is in heating-mode (fig. 3). When the green lamp is stable, the system has reached the set temperature and is ready to be used (fig. 4).
Sample preparation and loading:
- 12 µl lambda-DNA completely digested by HindIII, 4 µl EcoRI restriction enzyme, 4 µl 10 X EcoRI buffer and 20 µl Nuclease Free Water was added to an Eppendorf tube.
- The reaction mixture-tube was loaded into the block cooler for digestion at 37°C over night.
Electrophoresis and imaging:
- A 0.8 % agarose gel was prepared by adding 0.8 g agarose to 100 ml TBE solution and then boiling the solution in a microwave oven until the solution was clear. After cool-off to approximately 55 °C, the solution was poured into a pre-assembled GES-tray. The gel was left for polymerisation for about one hour.
- The digest-sample was taken from the block cooler, and 10 µl 5 X DNA sample loading dye was added.
- 1 µl 5X DNA sample loading dye was added to 4 µl 500 bp and 1 Kb DNA ladders.
- After polymerisation of the gel, it was mounted into the electrophoresis-tank and the submerged in TBE-buffer.
- A total of 0.5 µg of the digested samples and 0.2 µg of ladders were loaded (all volumes 12.5 µl) onto the gel.
- The electrophoresis was run as follows; 120 V for 90 minutes.
- After the electrophoresis, the gel was removed from the tank and the casting tray and soaked in 5 µg/ml ethidium bromide solution for 45 minutes with agitation in the dark.
- The gel was briefly soaked in ddH20 before visualisation and documentation.
- The gel was visualised in Dolphin-Doc Plus, using UV-as illumination source and a high transparent amber emission filter.
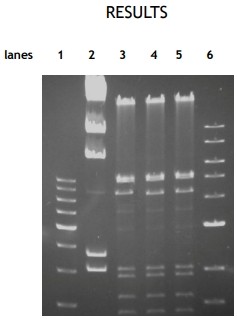
Figure 1: Restriction digest on agarose gel stained with ethidium bromide. Lane 1: 500 bp DNA-ladder (0.2 µg); lane 2: Lambda-DNA cut by HindIII (0.5 µg); lane 3, 4 and 5: Lambda-DNA cut by HindIII and EcoRI present different banding pattern compared with the template in lane 2; lane 6: 1 kb DNA-ladder (0.2 µg)
REMARKS
- The CB-1 block cooler provides a stable and reliable incubation system for restriction digestion applications. The temperature range is from 0°C to 75°C, and the following temperatures are available as pre-set; 4°C, 16°C, 25°C 37°C, 42°C, 55°C, 65°C and 72°C.
- A pre-warming/pre-cooling period of approximately 30 minutes guarantees an accurate and stable temperature. The block cooler is ready for use only when the green indicator is stable.
- In order to avoid unwanted degradation of DNA, only RNase and DNase-free water should be used. Degraded DNA would appear as a smear on the gel.
- In order to avoid “star”-activity of the restriction enzyme (altered or reduced activity), only use the correct buffer compatible with the enzyme with regards to salt concentration and pH. A concentrated buffer is often provided with the enzyme.
APPENDIX

Figure 2: Set one of the pre-programmed temperatures by pressing on the “up/down” arrows above “MODE” (green arrow). For fine-tuning or customised temperature setting, press the “Up/down” arrows above “TEMPERATURE” (red arrow). To start heating or cooling, press the “RUN/STOP”-button (blue arrow).

Figure 3: The display will show the set temperature (SET), as well as the current block temperature (BLK). When in heating mode, the red “heat”-indicator (red arrow), as well as the “power”-indicator (green arrow) are lit.

Figure 4: When the target temperature has been reached, as indicated by the display and the green “ready”-indicator glowing steadily (green arrow), the block cooler is ready to be used.
Joy Lee
Product Specialist
Email:
sales@wealtec.com
Wealtec BioScience Co. Ltd.
27Fl., No. 29-1, Sec. 2, Jungjeng E., Rd. Danshuei District, New Taipei City, Taiwan 25170
TEL: +886-2-8809-8587 FAX: +886-2-8809-8589;
http://www.wealtec.com
