Excellent Thermal Uniformity Showcase of the SEDI Thermal Cycler
Introduction
A good polymerase chain reaction (PCR) result is determined by several factors during the experiment. Factors such as the PCR setup, the quality of the reaction mix, and the thermal performance of the thermal cycler can greatly impact the efficiency and the quality of the PCR result. PCR itself is a dynamic temperature cycling process that is altering between different temperature stages: melting the target DNA sequences at ~95 °C, annealing primers to the single DNA strands at ~55-65 °C, and extending the primers to make new copies of the target DNA sequences at ~72 °C. To perform PCR process the temperature of the reaction block in a thermal cycler is cycled through these different temperatures and durations in a PCR protocol. Thus, the thermal uniformity of the reaction block controlled by a thermal cycler is the critical factor when assessing PCR result. Thermal uniformity is an essential characteristic of successful PCR assays. Uneven temperature in the reaction block negatively affects data accuracy and experimental reproducibility. For example, uneven hot spots cause the polymerase to denature and inactivate the PCR reaction, whereas uneven cold spots cause the primers to bind the unspecific sites of the DNA sequences, thus yield false positive results. A reliable thermal cycler with excellent temperature uniformity achieves the critical requirement to minimize the errors and produce accurate and reproducible PCR results. Here at Wealtec Bioscience, all of the SEDI thermal cyclers have been tested with a series of thermal evaluation and validation procedures before they are sent out to the customers. The evaluation and validation process implement the multi-channel temperature monitoring system that is specifically designed for assessing the performance of the dynamic temperature process of a thermal cycler. For monitoring the temperature of a thermal cycler, the temperature measuring protocol suggested by Span et al. (1) has been integrated to our standard evaluation and validation procedure. In addition to ensure that the SEDI can consistently match the performance, the plasmid DNA PCR reaction mix and agarose gel electrophoresis are used to further examine the actual PCR results.
Material and methods
Physical thermal evaluation
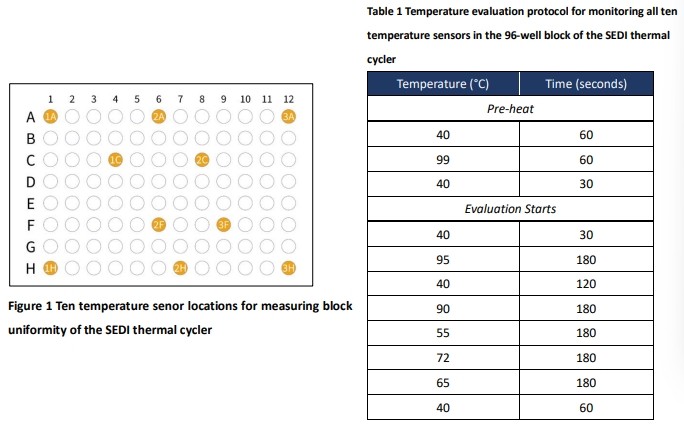
The data for assessing the thermal uniformity of the reaction block in the SEDI thermal cycler was recorded at six different temperature stages: 95, 90, 72, 65, 55, and 40 °C using ten temperature sensors and a data logger. Ten type-T microthermocouple sensors were connected to the multi-channel data logger (Graphtec GL220) and calibrated to the SI standard before probing in the 96-well reaction block of the thermal cycler. The data logger was capable of recording the temperature across the reaction block in a thermal cycler simultaneously in order to allow monitoring the uniformity. The ten sensor locations were probed in the wells of the 96-well reaction block as illustrated in Figure 1. The temperature measuring protocol illustrated in Table 1 is suggested by Span et al. (1) to record the six temperature that include both the pre-heat stage and evaluation stage. The recorded temperature data was acquired at five Hz, or five readings per second. In each well location where a sensor was probed, it had a half grain-size of thermal paste to ensure direct contact between the sensor and wall of the well. Temperature uniformity calculates the difference between the highest temperature and the lowest temperature measured by the sensors across the reaction block for each reading. When investigating the uniformity of a thermal cycler, the uniformity of the entire temperature protocol is examined, excluding the pre-heat stage. For the demonstration purpose, the heating and cooling phase were closely examined for the uneven temperature spots.
PCR amplification performance
The plasmid DNA PCR reaction mixes were prepared with the PCR mix for 20 μl reaction volume. For the PCR condition in the 20 μl reaction volume, 0.4 ng of the plasmid DNA pFLAG-mLRH-1, 0.125 μM of primers, 1× PCR mix (Solgent), and ultrapure water were all mixed together to a 1000 ul final mixture solution on the day of use. The final mixture solution was then aliquoted to 24 reaction tubes and placed in the SEDI thermal cycler in two fashions: the horizontal and diagonal fashion as illustrated in Figure 2. Each fashion was performed for two PCR conditions: the standard amplification and gradient method as listed in Table 2. For the horizontal fashion, the twelve reaction tubes were placed in row C, and the other 12 reaction tubes were placed in row F (Figure 2A). For the diagonal fashion, the twelve reaction tubes were placed from top left corner to bottom right corner, and the other 12 reaction tubes were placed from top right corner to bottom left corner (Figure 2B). Two amplification methods were performed, the standard methods and the gradient methods were programmed in the SEDI thermal cycler as listed in Table 2. The PCR products were separated through 1.5% agarose gel electrophoresis using the GES agarose gel electrophoresis unit and ELITE 600 power supply and then stained in ethidium bromide.
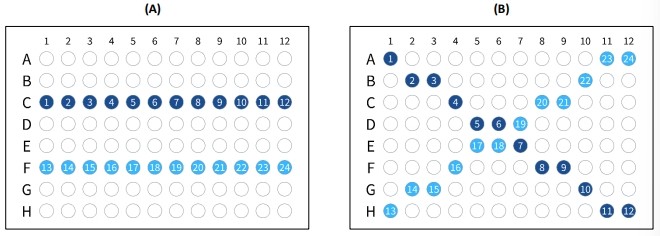
Figure 2 Twenty-four 20 μl PCR reaction tubes placement in 96-well reaction block in (A) horizontal and (B) diagonal fashion
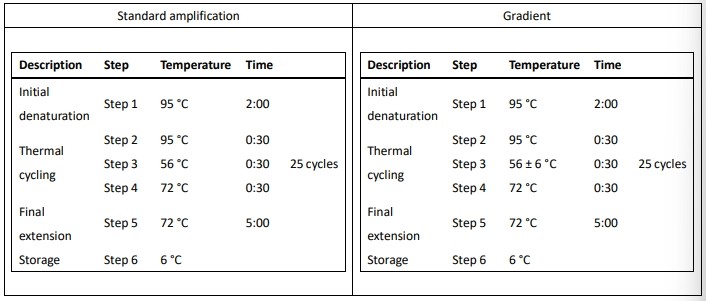
Table 2 Standard amplification and gradient PCR program for evaluating PCR performance of the SEDI thermal cycler
Results
Physical thermal evaluation
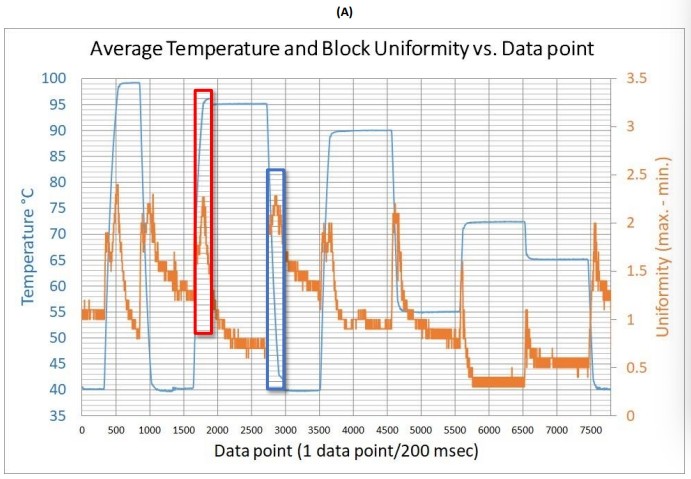
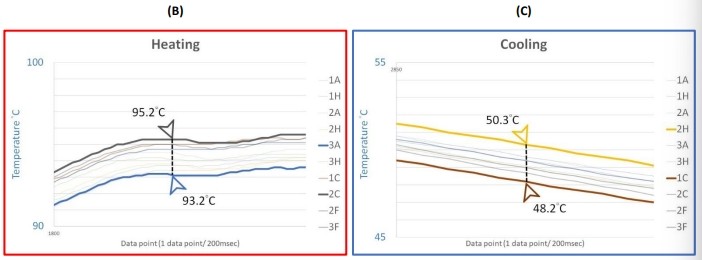
Figure 3 (A) Average temperature profile and block thermal uniformity of the SEDI thermal cycler measured by the 10-sensor multi-channel temperature monitoring system and the close examination of the temperature readings at the (B) heating phase and (C) cooling phase.
PCR amplification performance
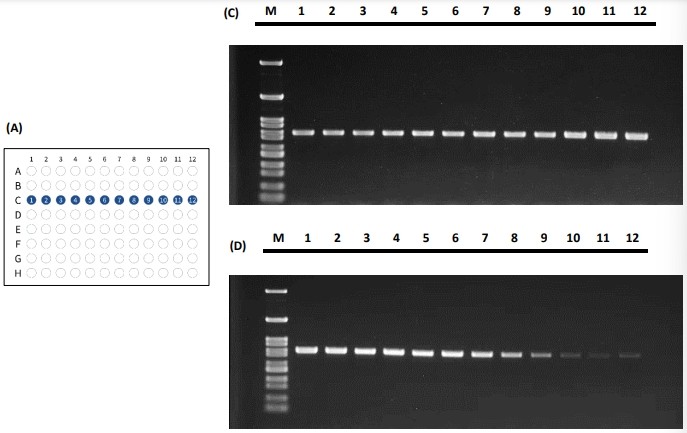
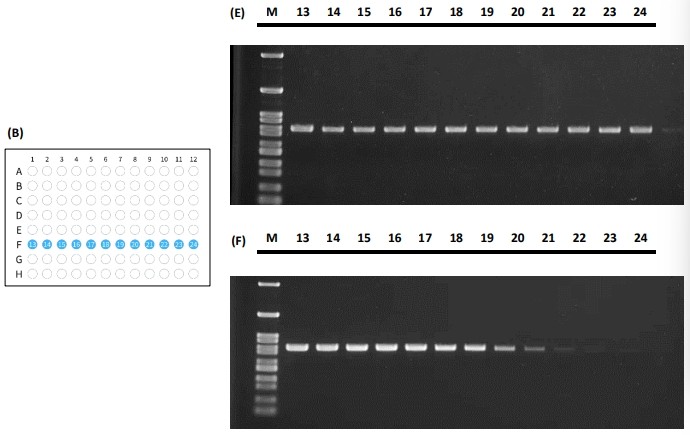
Figure 4 The PCR performance for tube placement in the horizontal fashion illustrated in (A) and (B) and its standard amplification performance shown in (C) and (E) and gradient performance shown in (D) and (F).
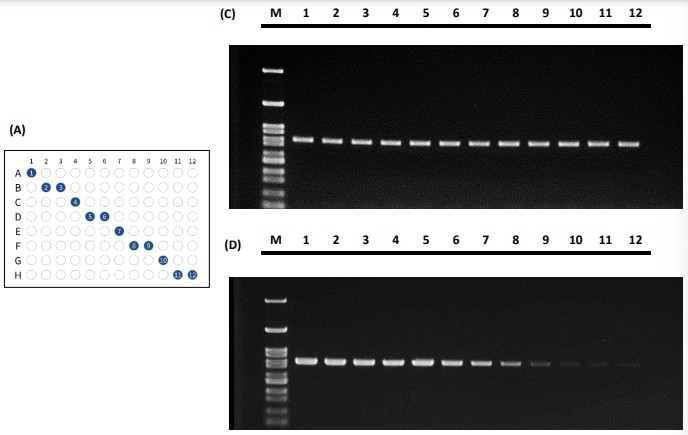
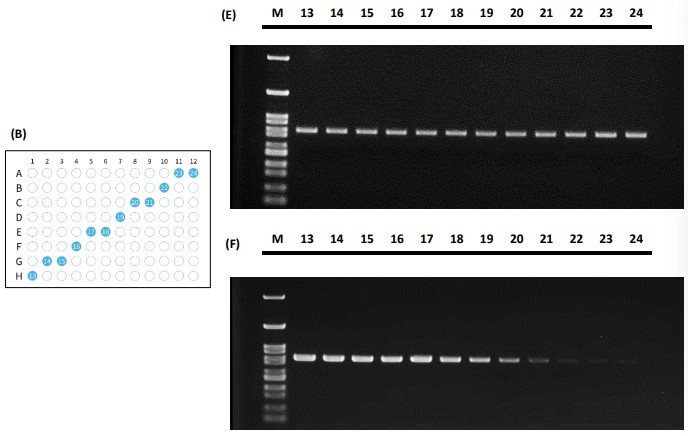
Figure 5 The PCR performance for tube placement in the diagonal fashion illustrated in (A) and (B) and its standard amplification performance shown in (C) and (E) and gradient performance shown in (D) and (F).
Discussion
Physical thermal evaluation
Given the entire average temperature and uniformity profile, the SEDI thermal cycler revealed its block uniformity when cycling through the 6 different temperature stages: 95, 90, 72, 65, 55, and 40 °C. Each peak in the uniformity plotting against data point chart in Figure 1A depicted which moment had the greatest differences between the two sensors measured by the data logger. When the reaction block in a thermal cycler was being heating up or cooling down, the non-uniformity was at its greatest. However, the SEDI thermal cycler not only keeps the block uniformity around 1 °C difference at the hold stages, but keeps it below 3 °C difference during the cooling and heating stages (Figure 1B and 1C). The SEDI can achieve consistent uniformity in the dynamic thermal process as a result of the high-accuracy calibration method and thorough testing.
PCR Performance
Both PCR tube placement fashions demonstrated that the actual PCR results yield consistent signal across the 96-well reaction block. The Peltier elements in the 96-well block tend to have the edge effect in which the edges have trouble balancing the temperature from the center. As the results shown in Figure 4 and Figure 5, the PCR results depicted excellent thermal uniformity and revealed no difference between the edge and middle during the cycling process in both standard amplification and gradient method.
Conclusion
The SEDI thermal cycler is characterized by its superb thermal uniformity which greatly contributes reproducibility in PCR results. The results in both the physical thermal evaluation and actual PCR demonstrated excellent uniformity and consistent PCR signals across the 96-well reaction block.
Ordering information
| Catalog no. | Description |
|---|---|
| 1310001 | SEDI B Thermal Cycler, gold-plated 96-well reaction module, 100-240V, 50-60 Hz |
| 1310006 | SEDI G Thermal Cycler, gradient unit, gold-plated 96-well reaction module, 100-240V, 50-60 Hz |
| 1310026 | SEDI X Thermal Cycler, gradient unit, gold-plated 384-well reaction module, 100-240V, 50-60 Hz |
| 1310061 | Step temperature control software with differential range ± 1-12 °C |
| 1311111 | Plate sealing machine |
| 1311112 | Sterilized sealing film, 100 sheets/package |
Joey Lai
Product Division
Email:
sales@wealtec.com
Wealtec BioScience Co. Ltd.
27Fl., No. 29-1, Sec. 2, Jungjeng E., Rd. Danshuei District, New Taipei City, Taiwan 25170
TEL: +886-2-8809-8587 FAX: +886-2-8809-8589;
http://www.wealtec.com
